zinc valence electrons|how to calculate valence electrons : Baguio Zinc (Zn) Zinc is the 30th element in the periodic table and has a symbol of Zn and atomic number of 30. It has an atomic weight of 65.38 and a mass number of 64. Zinc has thirty .
XNXX.COM 'bbw hypnotized' Search, free sex videos
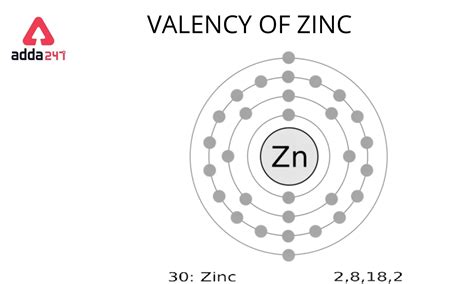
zinc valence electrons,Mar 23, 2023 Zinc has 2 valence electrons, both located in the 4s-orbital. The electron configuration of a neutral zinc atom is Zn:1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 or, if you use the noble gas shorthand notation .

Zinc has a valence of +2, meaning it can form compounds with two . 156. 18K views 1 year ago. To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal . Valency of Zinc. The precise and accurate valency of Zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. The outermost .Zinc (Zn) Zinc is the 30th element in the periodic table and has a symbol of Zn and atomic number of 30. It has an atomic weight of 65.38 and a mass number of 64. Zinc has thirty .Learn how to use the periodic table to determine the number of valence electrons for main group elements. Zinc is in group 12 and has two valence electrons.Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are .
how to calculate valence electrons The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the .Overview Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have . In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for .
The zinc atom donates two electrons in the 4s orbital to form a zinc ion (Zn 2+ ). Zn – 2e – → Zn 2+. Here, the electron configuration of zinc ion (Zn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. Zinc atom exhibits +2 .
The Valence Orbital Diagram for Zinc (Zn) Zinc (Zn) is a transition metal that has a valence electron configuration of [Ar] 3d 10 4s 2. This means that in its ground state, zinc has two valence electrons. The valence orbital diagram is a way to represent these electrons in their respective energy levels and orbitals.Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of .
Zinc Electron Configuration. The Mg 2+ and Zn 2+ ions are of the same size. Zinc is the 24th most abundant element present in the Earth’s crust. It has five stable isotopes. The common zinc ore is sphalerite or zinc blende, which is a zinc sulfide mineral. The largest working lodes of Zinc are in Asia, Australia, and the United States. 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..zinc valence electrons how to calculate valence electrons 2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr. La tabla periódica muestra que los números atómicos del zinc (Zn) son 30. Esto significa que el número total de electrones en el átomo de zinc es treinta. Los términos ” grado de oxidación ” y ” valencia ” pueden no ser los mismos, pero son numéricamente casi idénticos.Zinc protons, neutrons and electrons. Thus, the number of neutrons in an element is obtained from the difference between the number of atomic masses and the number of atoms. That is, neutron number (n) = atomic .

Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
zinc valence electrons Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .How many valence electrons does zinc have? Open in App. Solution. Verified by Toppr. The electronic configuration of zinc is $$1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10}$$ As n=4 is the valence shell. So, the no. of valence electrons in zinc are 2. Was this answer helpful? 4. Similar Questions. Q1.
In the case of titanium, it has an atomic number of 22, which means it has 22 electrons. The electronic configuration of titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 2. The valence electrons of an atom are the electrons in the outermost energy level or shell.Element Zinc (Zn), Group 12, Atomic Number 30, d-block, Mass 65.38. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined). The completely filled d orbitals count as core, not valence, electrons. Transition elements or transition metals.
1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's .
And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system .
zinc valence electrons|how to calculate valence electrons
PH0 · valence orbital diagram zn
PH1 · valence electrons chart
PH2 · periodic table with valence electrons
PH3 · orbital diagram for zn
PH4 · list of valence electrons for each element
PH5 · how to calculate valence electrons
PH6 · how many electrons does zinc have
PH7 · electron configuration of zn
PH8 · Iba pa